how to draw molecular orbital diagram of no
The first two to go are from the 4s orbital and Cobalt becomesAr4s 0 3d 7. A molecular orbital MO diagram explains the chemical bonding in molecules by energy level diagrams.
Explain The Mo Diagram For No Molecule Sarthaks Econnect Largest Online Education Community
Now carbon monoxides MO.
. We can take care of your. It has two equal NO bonds of 125 pm intermediate in length. There are a variety of types of forces.
Sp 3 An example of this is methane CH 4. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. An egg is free-falling from a nest in a tree.
TikZ-Feynman is a LaTeX package allowing Feynman diagrams to be easily generated within LaTeX with minimal user instructions and without the need of external programsIt builds upon the TikZ package and its graph drawing algorithms in order to automate the placement of many verticesTikZ-Feynman still allows fine-tuned placement of vertices so that even complex. As we know N2O4 molecule is a dimer of the NO2 molecule hence well discuss molecular orbital diagram of NO2 molecule first. The terms atomic orbital and molecular orbital were introduced by Robert S.
The 3s group orbitals of chlorine atom will remain non-bonding because their energy is very low as compared to the 2s and 2p atomic orbitals of. Solution We draw a molecular orbital energy diagram similar to that shown in. To do this we use the draw command followed by by some additional arguments.
O 2 molecule contains one unpaired electron in each of one π2p x and π2p y orbitals. Of valence electrons is 22. Previously in this lesson a variety of force types were placed into two broad category headings on the basis of whether the force resulted from the contact or non-contact of the two interacting objects.
Weve also specified the colour gray and told it to make the lines very thinAfter these arguments we enter the co-ordinates of the bottom-left corner followed by the. Course help online is here to help with such urgent orders. N2O4 Molecular Orbital MO Diagram.
Xenon difluoride is a noble gas hypervalent halogen compound with an exception in the octet rule and no net dipole moment. Mulliken in 1932 to mean. A force is a push or pull acting upon an object as a result of its interaction with another object.
N 2 2- N 2- N 2 N 2. Explain on the basis of the molecular orbital diagram why O 2 should be paramagnetic. First let us know what molecular orbital diagram is.
This is the combination of one s orbital and two p orbitals. Each oxygen atom contributes six electrons so the diagram appears as shown in. Very often when drawing diagrams we will want to draw a grid.
First look for the total number of valence electrons in a single sulfur trioxide SO3 molecule which is twenty-four. From this diagram calculate the bond order for O 2. Then the next electron leaves the 3d orbital and the configuration becomes.
Diagram the forces acting on the egg as it is falling. The molecular orbital diagram of BeCl2 will be drawn by combining atomic orbitals of beryllium atom and group orbitals of chlorine atom having similar energy and symmetry around a molecular axis. They were proposed by Robert S.
Considering the energy level diagram the configuration of N2 is σ1S2 σ 1S2 σ2S2 σ2S2 π2Px2 π2Py2 σ2Pz1. The atmosphere of Earth or air is the layer of gases retained by Earths gravity that surrounds the planet and forms its planetary atmosphereThe atmosphere of Earth protects life on Earth by creating pressure allowing for liquid water to exist on the Earths surface absorbing ultraviolet solar radiation warming the surface through heat retention greenhouse effect and reducing. Ar4s 0 3d 6.
We have qualified academic writers who will work on your agent assignment to develop a high quality paper for you. For instance in NO 2 nitrite anion the two NO bond lengths are equal even though no single Lewis structure has two NO bonds with the same formal bond orderHowever its measured structure is consistent with a description as a resonance hybrid of the two major contributing structures shown above. Draw the molecular orbital diagram for the oxygen molecule O 2.
Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. Likewise the orbital correlation diagram for methane provides another example of the difference in electron density predicted by molecular orbital calculations from that of the localized bond model. In chemistry a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a moleculeThis function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region.
Define antibonding molecular. This is the combination of one s orbital and three p orbitals. It depends on the octet rule concept and is an extension of the electron dot diagram.
The herbicide propyzamide increases inflammation in the small and large intestine and the AHRNF-κBCEBPβ signalling axiswhich operates in T cells and dendritic cells to promote. Sp 2 An example of this is ethylene C 2 H 4. A resistor a variable resistor a transmission line a closing switch a european current source a european voltage source an empty diode a full led a generic bipole and a sinusoidal voltage source.
Here the correlation diagram correctly accounts for the paramagnetic character of this simple diatomic compound. The water cycle also known as the hydrologic cycle or the hydrological cycle is a biogeochemical cycle that describes the continuous movement of water on above and below the surface of the EarthThe mass of water on Earth remains fairly constant over time but the partitioning of the water into the major reservoirs of ice fresh water saline water salt water and atmospheric. Thus to have a comprehensive idea about CS2 Lewis Structure let us go through each step clearly and systematically.
Click on the compound names for. Mulliken and Friedrich Hund in 1928. Arrange the following molecular species in increasing order of stability.
Mixing of MOs results in the development of anti-bonding orbitals that give us 11 filled molecular orbitals also we know XeF2 follows a 3-center-4-electron bond model. When two orbitals are added the result is stable bonding molecular orbital and when orbitals are subtracted it is called unstable anti-molecular bonding which has more energy than the latter one. A flying squirrel is gliding no wing flaps from a tree to the ground at constant velocity.
Now as most of us are aware a molecular orbital is a function to understand the location and wave-like nature thus a delocalized approach to bonding. These examples are all bipoles. In the case of I3- the no.
Diagram the forces acting on the combination of gymnast and bar. All you have to do is chat with one of our online agents and get your assignment taken care of with the little remaining time. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO molecular orbital method in particular.
A MO can have a maximum of 2 electrons. From the bottom left we have. Thus we can see that there are six electrons that need to be apportioned to Crystal Field Diagrams.
When observing Cobalt 3 we know that Cobalt must lose three electrons. The Lewis structure of sulfur trioxide SO3 molecule is drawn by. For example we specify the grid step size using step and a length.
How does this diagram account for the paramagnetism of O 2. This combines one s orbital with one p orbital. This means that the s and p characteristics are equal.
MO results from overlapping between AOatomic orbitals of the same energy. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing.
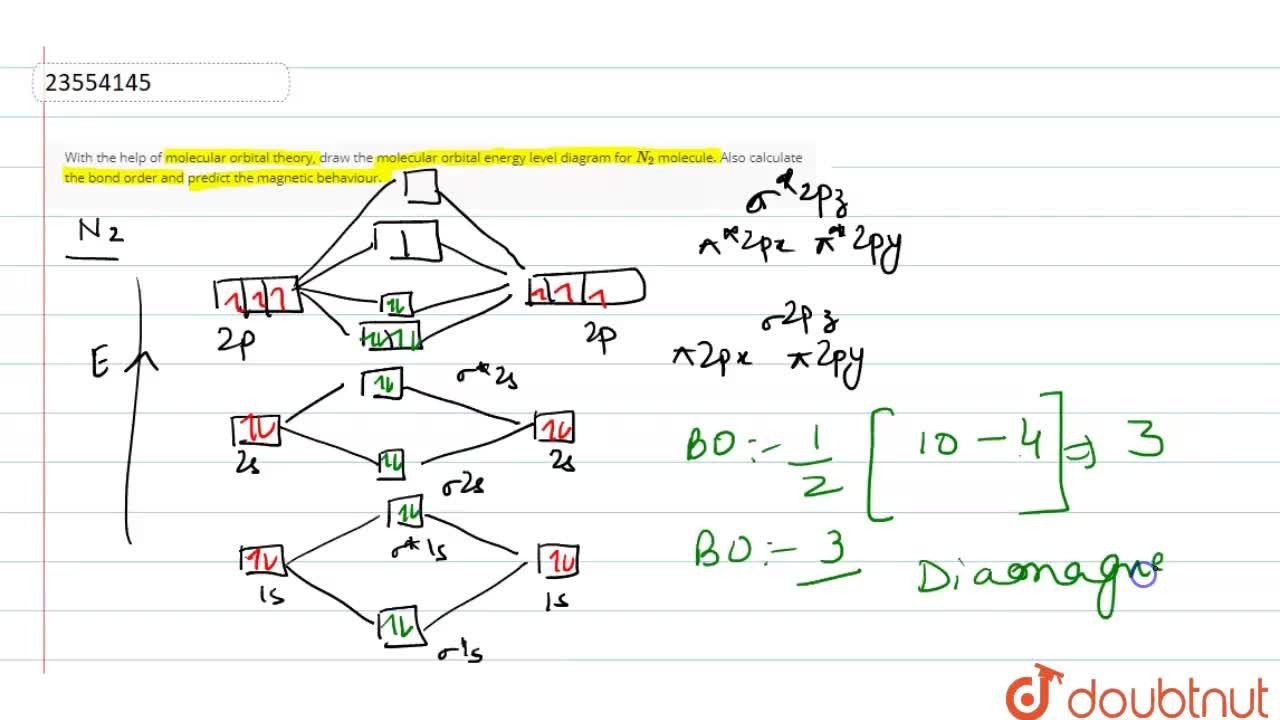
With The Help Of Molecular Orbital Theory Draw The Molecular Orbital Energy Level Diagram For N 2 Molecule Also Calculate The Bond Order And Predict The Magnetic Behaviour

8 5 Molecular Orbital Theory Chemistry
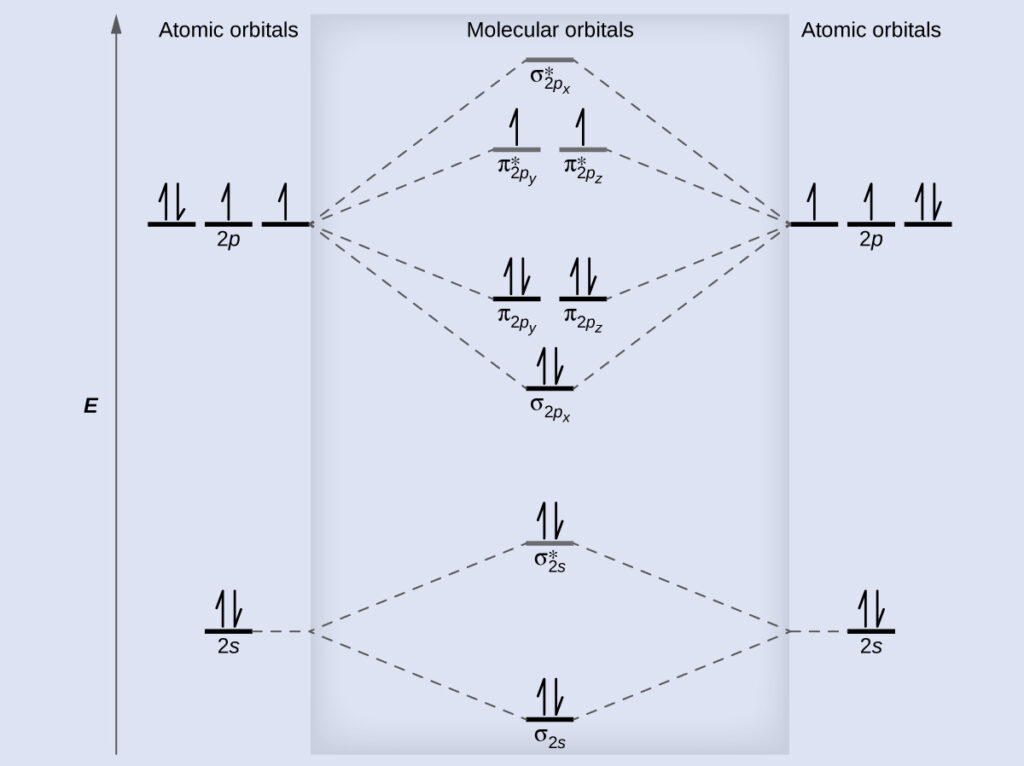
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Ucalgary Chem Textbook
Consider The Following Molecules No No And No Using The Molecular Orbital Theory How Do You Evaluate Them In Terms Of Bond Energy And Stability Quora
Molecular Structure Atomic Orbitals
What Is The Molecular Orbital Diagram For No Quora

Molecular Orbital Diagram Of B2 C2 And N2 Molecules Youtube

Draw The Molecular Orbital Energy Level Diagram Of N2 Molecules
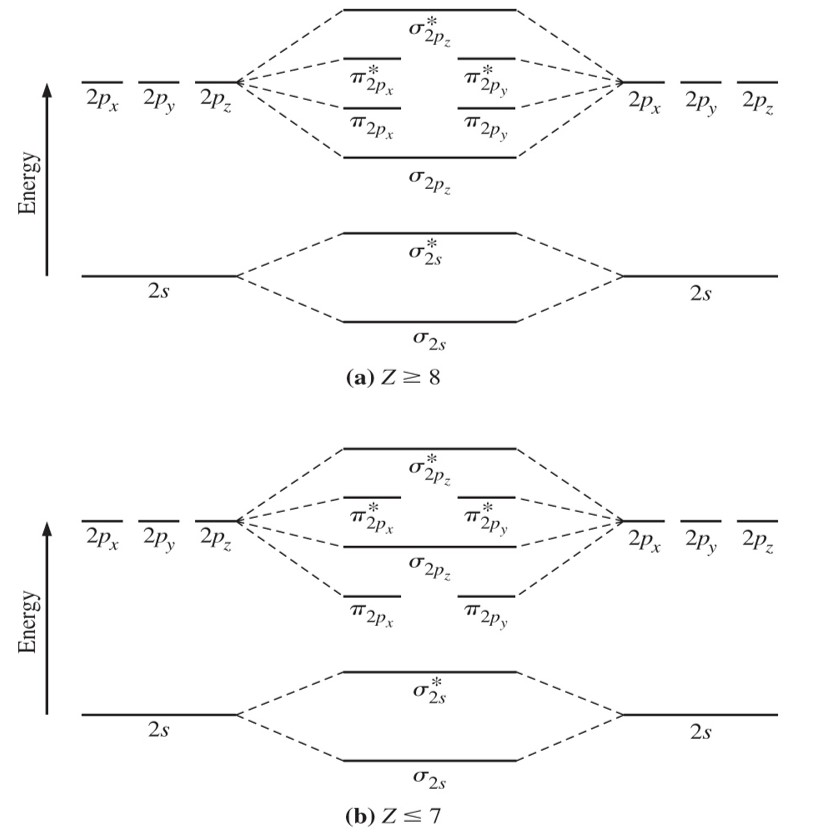
Solved 1 A Draw The Molecular Orbital Energy Level Scheme Chegg Com
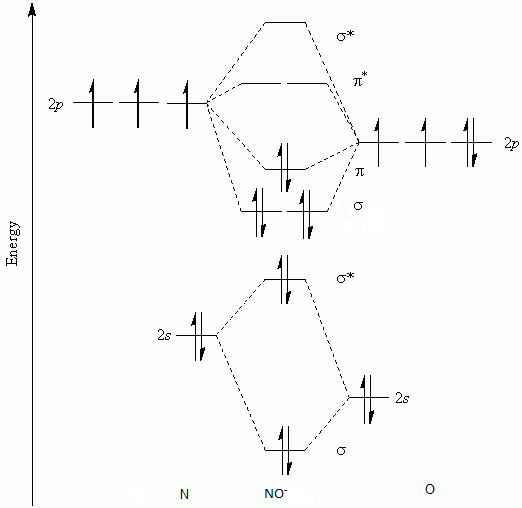
What Is The Bond Order Of No Class 12 Chemistry Cbse

Molecular Orbital Diagram For No Download Scientific Diagram

Glv Tutoring Figure Molecular Orbital Diagram For O2 Molecular Orbital Diagrams Are Experimentally Quantified Diagrams That Measure Electron Distribution Amongst Its Possible Quantum States One Way To Think About Molecular Orbital
Molecular Orbitals Introductory Chemistry

Molecular Orbitals Of No No And No A Computational Quantum Chemistry Experiment Journal Of Chemical Education

Molecular Orbital Diagram Wikipedia
Draw Mo Diagram Of Co And Calculate Its Bond Order Sarthaks Econnect Largest Online Education Community

The Pi Molecular Orbitals Of Benzene Master Organic Chemistry

Molecular Orbitals In Chemical Bonding Ppt Video Online Download
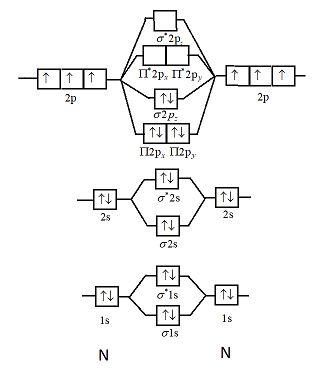
Draw The Molecular Orbital Diagram Of N2n2 N2 Write Class 11 Chemistry Cbse